Retinopatia por Tamoxifeno. Uma Complicação Incomum, mas Grave: Relato de Caso
Conteúdo do artigo principal
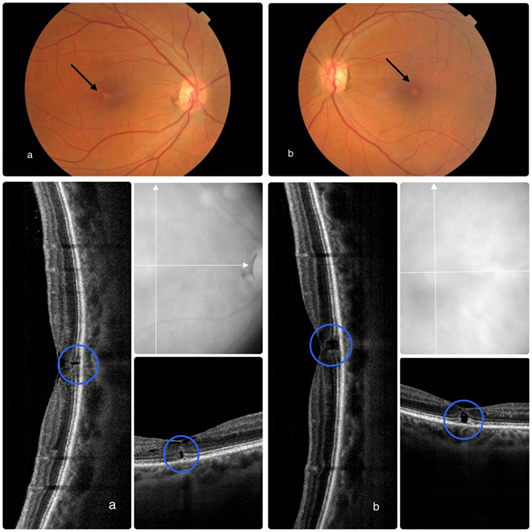
Resumo
Detalhes do artigo

Este trabalho está licenciado sob uma licença Creative Commons Attribution 4.0 International License.
Authors retain the copyright of their articles and grant the journal the right of first publication under the Creative Commons Attribution (CC BY) license, which allows others to share and adapt the work with proper attribution.
Referências
Tamoxifen for early breast cancer: an overview of the randomised trials. Early Breast Cancer Trialists' Collaborative Group. Lancet. 1998 May 16;351(9114):1451-67.
Kaiser-Kupfer MI, Lippman ME. Tamoxifen retinopathy. Cancer Treat Rep. 1978 Mar;62(3):315-20.
Noureddin BN, Seoud M, Bashshur Z, Salem Z, Shamseddin A, Khalil A. Ocular toxicity in low-dose tamoxifen: a prospective study. Eye (Lond). 1999 Dec;13 (Pt 6):729-33. doi: 10.1038/eye.1999.217.
Davies C, Pan H, Godwin J, Gray R, Arriagada R, Raina V, Abraham M, Medeiros Alencar VH, Badran A, Bonfill X, Bradbury J, Clarke M, Collins R, Davis SR, Delmestri A, Forbes JF, Haddad P, Hou MF, Inbar M, Khaled H, Kielanowska J, Kwan WH, Mathew BS, Mittra I, Müller B, Nicolucci A, Peralta O, Pernas F, Petruzelka L, Pienkowski T, Radhika R, Rajan B, Rubach MT, Tort S, Urrútia G, Valentini M, Wang Y, Peto R; Adjuvant Tamoxifen: Longer Against Shorter (ATLAS) Collaborative Group. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen re-ceptor-positive breast cancer: ATLAS, a randomised trial. Lancet. 2013 Mar 9;381(9869):805-16. doi: 10.1016/S0140-6736(12)61963-1.
Tenney S, Oboh-Weilke A, Wagner D, Chen MY. Tamoxifen retinopathy: A comprehensive review. Surv Ophthalmol. 2024 Jan-Feb;69(1):42-50. doi: 10.1016/j.survophthal.2023.07.003.
Wang L, Miao H, Li X. Tamoxifen retinopathy: a case report. Springerplus. 2015 Sep 17;4:501. doi: 10.1186/s40064-015-1258-2..
Doshi RR, Fortun JA, Kim BT, Dubovy SR, Rosenfeld PJ. Pseudocystic foveal cavitation in tamoxifen retinopathy. Am J Op-hthalmol. 2014 Jun;157(6):1291-1298.e3. doi: 10.1016/j.ajo.2014.02.046.
Heier JS, Dragoo RA, Enzenauer RW, Waterhouse WJ. Screening for ocular toxicity in asymptomatic patients treated with tamoxifen. Am J Ophthalmol. 1994 Jun 15;117(6):772-5. doi: 10.1016/s0002-9394(14)70321-6.
Vinding T, Nielsen NV. Retinopathy caused by treatment with tamoxifen in low dosage. Acta Ophthalmol (Copenh). 1983 Feb;61(1):45-50. doi: 10.1111/j.1755-3768.1983.tb01393.x.
Kim HA, Lee S, Eah KS, Yoon YH. Prevalence and Risk Factors of Tamoxifen Retinopathy. Ophthalmology. 2020 Apr;127(4):555-557. doi: 10.1016/j.ophtha.2019.10.038.
Ahn SJ, Kim J, Kwon HY. Nationwide Screening Practices for Tamoxifen Retinal Toxicity in South Korea: A Population-Based Cohort Study. J Clin Med. 2024 Apr 9;13(8):2167. doi: 10.3390/jcm13082167.
Li C, Xiao J, Zou H, Yang B, Luo L. The response of anti-VEGF therapy and tamoxifen withdrawal of tamoxifen-induced cystoid macular edema in the same patient. BMC Ophthalmol. 2021 May 7;21(1):201. doi: 10.1186/s12886-021-01953-z.
Koulisis N, Moysidis SN, Olmos de Koo LC, Russell CA, Kashani AH. The tipping point: Tamoxifen toxicity, central serous chorioretinopathy, and the role of estrogen and its receptors. Am J Ophthalmol Case Rep. 2016 May 18;3:8-13. doi: 10.1016/j.ajoc.2016.05.004.
Park YJ, Lee S, Yoon YH. One-year follow-up of optical coherence tomography angiography microvascular findings: macular telangiectasia type 2 versus tamoxifen retinopathy. Graefes Arch Clin Exp Ophtha.lmol. 2022 Nov;260(11):3479-3488. doi: 10.1007/s00417-022-05695-6.
Kiranmayee, Sai P; Kalluru, Viswanath; Govindahari, Vishal. Tamoxifen maculopathy – A case with early optical coherence tomography changes. Indian J Ophthalmol - Case Reports 2(2):p 463-464, Apr–Jun 2022. Doi: 10.4103/ijo.IJO_2350_2.